
Impacts of Canada’s changing climate on West Nile Virus vectors

Key Messages |
|
Introduction
West Nile Virus (WNV) is a mosquito-borne disease that spread to Canada in 2002. Since then, it has become endemic in many parts of the country and is the most common mosquito-borne disease in Canada. Human cases of the disease vary significantly from year to year, and typically occur in the summer or early autumn.1 While most people (70–80%) infected with WNV experience no symptoms, others have symptoms that range from mild to severe, and in select cases, even result in death.2 The most common symptoms include fever, body aches, and fatigue, among others. In the case of severe disease (also called neuro-invasive disease), symptoms may also include stiff neck, disorientation, convulsions, and paralysis. While WNV affects only a small proportion of the human population in Canada annually, cases are expected to increase given the impacts of climate change on mosquito vector distribution and disease transmission dynamics.1,3-9 Already, the effects of climate change on WNV are reflected in newly established mosquito populations and disease patterns.10,11 As the planet continues to warm, infections are expected to increase throughout the 21st century, with one study predicting a nearly doubling of cases of WNV neuro-invasive disease in the US by the mid 21st century.3 It is also likely that new invasive species of WNV and other vector-borne diseases will continue to be introduced to Canada as a result of both globalization and climate change.12,13
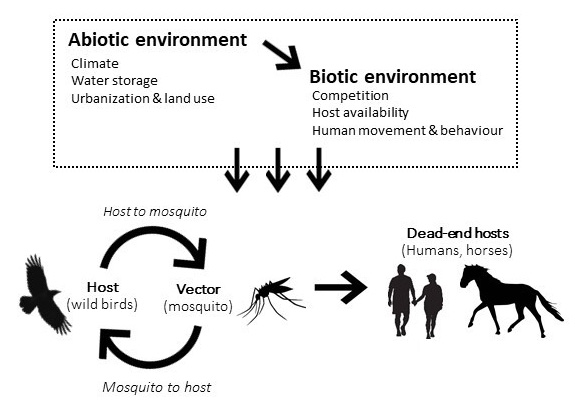
The virus is maintained in nature in a cycle between mosquitoes and avian hosts, and transmission of the virus is heavily influenced by both abiotic (e.g., climate, water storage, urbanization, and land use) and biotic factors (e.g., competitors, host availability, human movement) (Figure 1). Though the predominant host reservoir for WNV is birds, mosquitoes as vectors can also transmit the virus to humans and other mammals, which are considered dead-end hosts as they are unable to transmit the virus themselves due to insufficient viremia.
Figure 1. WNV transmission pathway and the influence of abiotic and biotic factors
Over 65 mosquito species have been shown to be vectors of WNV globally;14 however, in Canada, the predominant WNV vectors are Culex (Cx.) pipiens, Cx. restuans, and Cx. tarsalis.11,14-16 These species have historically played an important role in WNV transmission in Canada. Cx. pipiens and Cx. restuans are well adapted to urban environments and are the predominant WNV vectors in Eastern Canada. Because they are difficult to tell apart visually, they are often referred to together as Cx. pipiens-restuans. Conversely, Cx. tarsalis prefer grassland and agricultural areas and are the predominant vector in the Prairies and Western Canada. Another species—Aedes (Ae.) albopictus—is newly established in Canada, as of 2016. So far, this species has only been found in southwestern Ontario; however, it is a competent vector of WNV, among other pathogens, and is predicted to expand further into Canada due to climate change.[1] Like Cx. pipiens-restuans, it prefers urban environments.
To date, many studies have investigated the association between climate variables with human cases of WNV, mosquito infection prevalence, and vector abundance. These correlative studies have produced a range of results, highlighting the complexity of virus-vector-host interactions, especially across different mosquito species and regions of the world. Several species’ distributions models have also been used to predict a climate-change–driven range expansion of the principal West Nile virus vectors.17-22 This review will summarize the current and projected distributions of Cx. pipiens, Cx. restuans, Cx. tarsalis, and Ae. albopictus in Canada under a changing climate, and discuss how climate change may impact WNV outbreaks in humans in the future. This review will also discuss considerations for municipal mosquito control programs in response to these impacts, including in those regions where WNV is likely to become endemic.
[1] Another possible competent WNV vector in Canada includes Ae. japonicus. This species is a competent vector of WNV in the lab, and may contribute to WNV spread in the field, though investigations are ongoing.
Methodology
Literature search
We searched the scholarly and grey literature for information on the four confirmed WNV vectors (Cx. pipiens, Cx. restuans, Cx. tarsalis, and Ae. albopictus), West Nile Virus, and the impacts of climate change using the EBSCOhost databases (includes Medline, CINAHL, Academic Search Complete, and Eric), Google Scholar and Google. No limitations were placed on date of publication. All relevant English-language results were collated and additional documents identified through forward and reverse citation chaining, as well as through expert consultation. Complete search terms and the full list of results are available upon request.
Studies were selected for review if they reported on
- the current distribution in Canada of one or more of the four mosquitoes being discussed in this paper;
- the projected distribution due to climate change in Canada of one or more of the four mosquitoes being investigated; and
- the impacts of climate change (warmer temperatures, increased/decreased precipitation, and extreme weather events) on human cases of WNV (in Canada or otherwise).
One review article from 2015 looked extensively at the impacts of climate change on WNV transmission in a global context,1 and another two reviews examined environmental determinants and risk factors for avian-associated mosquito-borne diseases in Canada.4,23 Accordingly, the objective of this review was to focus specifically on WNV in the Canadian context, with a focus on synthesizing projected distributions of predominant WNV mosquito vectors and bringing up to date previous syntheses of climate-change-driven impacts.
A single reviewer assessed the studies and the results were synthesized narratively. The synthesis was subject to internal and external review.
Findings
What is the current distribution of Cx. pipiens, Cx. restuans, Cx. tarsalis and Ae. albopictus in Canada?
Mosquito vectors of WNV are limited to latitudes and altitudes where winters are short enough for them to survive.24 Until recently, available mosquito distribution maps at the national level were dated, of low resolution, and based on published occurrence records dating back to the 1950s.24-26 Maps by Darsie and Ward (2005) show the estimated distribution of Cx. pipiens, Cx. restuans, and Cx. tarsalis (Figure 2). Darsie and Ward do not mention Ae. albopictus in their compendium. The species spread to North America in 1985 and was first detected in Canada in 2002, although it was not confirmed to have been established until more recently.10
Figure 2. Map of estimated distribution of Cx. pipiens, Cx. restuans, and Cx. tarsalis

Adapted from Darsie and Ward (2005)24
More recent efforts have been made to update this information using developments in mathematical ecology, which offer statistical methods to model the habitat suitability of mosquitoes when presence records are available. These models use a variety of methods, but typically rely on mosquito surveillance data and remotely sensed environmental covariates, allowing for a more practical way to estimate species distribution without having to systematically sample large areas for species presence.
For Cx. tarsalis, two habitat suitability models have been developed by Chen et al. (2013) and Gorris et al. (2021).17,27 These studies indicate habitat suitability in similar areas to the range proposed by Darsie and Ward for Alberta and Saskatchewan. However, in Manitoba, both studies suggest habitat suitability is restricted to the southwest of the province because of a lack of suitable grassland habitat elsewhere. Records of Cx. tarsalis have also been found in the Northwest Territories, and for the first time in the Yukon.28,29
For Cx. pipiens, two habitat suitability models have been developed by Hongoh et al. (2012) and Gorris et al. (2021).18,27 These studies show habitat suitability consistent with Darsie and Ward’s proposed distribution but identify additional suitable habitat for Cx. pipiens in northern Ontario (along the Manitoba border), as well as southern Alberta and Saskatchewan. Hongoh et al. (2012), however, determine habitat suitability in Alberta and Saskatchewan somewhat more conservatively than Gorris et al. (2021). Gorris et al. (2021) further identify southern Manitoba as having suitable Cx. pipiens habitat, while Hongoh et al. (2012) do not.27 Records of Cx. pipiens have also been reported in Winnipeg, Manitoba,[a] and northern BC in Valemount and Prince George.30 These records are much further north than expected; however, it is suspected that these mosquitoes are able to either overwinter in heated man-made structures or animal-made structures (e.g., animal burrows), thus allowing their survival.
For Cx. restuans, only one habitat suitability model has been developed.27 Gorris et al (2021) identify high habitat suitability in southern Ontario and Quebec—consistent with the range proposed by Darsie and Ward— but, much lower habitat suitability in the Prairie provinces. While this remains consistent with what has been proposed by Darsie and Ward, it should be noted that habitat suitability in this area remains comparatively low. More research is needed to confirm habitat suitability for this species.
Finally, habitat suitability models have also been developed for Ae. albopictus.19-22,31,32 Habitat suitability is consistently suggested along the BC coastline, including Vancouver Island,19-22 southwestern Ontario,19,20,22,31,32 southern Quebec,19,21,32 and southern New Brunswick.19,21,22,32 A few studies have also suggested suitability in Nova Scotia,21 Newfoundland and southern Labrador,21 southeastern Ontario,19,32 and southwestern Alberta.22 To date, records of Ae. albopictus have only been found in southwestern Ontario; however, the species has also been reported in Seattle, which is approximately 150 km from the Canadian border, suggesting risk is not limited to southwestern Ontario.10
[a] Email communication with A. Sarkar, PhD, Memorial University, St. John’s, NL, Oct 4, 2022.
What are the impacts of Canada’s changing climate on the future distribution of Cx. pipiens, Cx. restuans, Cx. tarsalis and Ae. albopictus?
According to multiple lines of evidence, it is virtually certain that Canada’s climate has warmed and will continue to warm into the future if global greenhouse gas emissions continue unabated.33,34 Climate models project annual and seasonal mean temperature increases across Canada, with pronounced change occurring in northern Canada, particularly during winter months.33 The annual number of hot days is projected to increase, with Ontario seeing the largest increase in the number of hot days overall. As temperatures become warmer, precipitation will continue to shift from snow to rain in the spring and fall seasons. There is medium confidence that annual and winter precipitation is projected to increase across Canada, with the largest changes to be experienced in northern Canada.33 Daily extreme precipitation is also expected to increase. Nonetheless, summer precipitation is projected to decrease across much of southern Canada, though confidence in this projection is not as high as for annual mean precipitation. While the intensity and frequency of one-day heavy precipitation events have very likely increased since the mid-20th century across most of the US, no detectable trend has been observed for Canada.33 However, recent flooding events along the Pacific Coast have been linked to increasingly intense atmospheric river events.
For Cx. pipiens, Cx. restuans, Cx. tarsalis and Ae. albopictus, these temperature and precipitation shifts are important predictors of their distribution.28,32,33 There is already evidence that the range distribution of these species has begun to shift, increasing the risk of WNV becoming established in new places. Warmer annual and seasonal temperatures have and will continue to increase the geographic spread of where mosquitoes can survive and breed.1,9,35 The role of precipitation is less straightforward and more species-specific. While increased rainfall increases the amount of standing water necessary for breeding, heavy rainfall can also dilute nutrients in the standing water required for larval survival or flush out ditches and drainage channels that had served as breeding grounds.36,37 In times of decreased precipitation or drought, standing water conditions can become richer in organic material needed for larval survival, and have been observed to lead to population outbreaks of some species of mosquito, such as Cx. pipiens, in the following year.38,39
To forecast where these four mosquito species are likely to expand to in the future, models that account for the impacts of climate change on future habitat suitability have been helpful. However, research in this area remains sparse, and only a handful of studies have examined climate change impacts on WNV vectors in Canada.17-22 For both Cx. tarsalis and Cx. pipiens, there is only one predictive model each of future habitat suitability in Canada. For Ae. albopictus, there are four predictive models, two of which examine habitat suitability globally; the remaining two studies examine habitat suitability for the US and Canada specifically. These six models have been developed using a wide range of methods. The more recent studies have used Representative Concentration Pathways (RCPs) from the Intergovernmental Panel on Climate Change‘s (IPCC) fifth assessment report, while older studies used emission scenarios from IPCC’s third and fourth assessment reports (Appendix A). A variety of general circulation models (GCMs) and regional circulation models (RCMs) have been used to construct these scenarios (Table A1). No studies have investigated the impacts of climate change on future habitat suitability for Cx. restuans.
For Cx. tarsalis, Chen et al. (2013) modelled habitat suitability in the Prairie provinces using future climate conditions that were either: 1) cool and wet, 2) median, or 3) warm and dry.17 Three time slices were modelled: the 2020s (2010–2039), the 2050s (2040–2069), and the 2080s (2070–2099). Except for the cool and wet scenario in the 2020s, Cx. tarsalis is predicted to expand northward under all future scenarios and time slices. Under the median scenario, this amounts to a 1.3-fold increase in geographical range for the 2020s, a 1.6-fold increase for the 2050s, and a 1.9-fold increase for the 2080s. While climate conditions in the northern parts of the Prairies (up to 60° N latitude) were found to be increasingly suitable under future climate scenarios, Cx. tarsalis range expansion will be primarily restricted by the absence of suitable grassland habitat.
For Cx. pipiens, Hongoh et al. model habitat suitability across Canada (excluding BC and the Territories) under a moderate (1.0–2.2° C) and more extreme warming (2.0–5.4° C) scenario for the 2020s (2011–2040), 2050s (2041–2070) and 2080s (2071–2100).18 Under the moderate scenario, the increase of suitable habitat range is initially concentrated in southern Ontario, parts of southern Quebec, and parts of the Maritime provinces (New Brunswick, Nova Scotia, and Prince Edward Island) as well as Newfoundland and Labrador. Suitable habitat range expansion continues further northward and westward into central Ontario, southern Quebec, and a larger region of New Brunswick and Newfoundland by the 2050s. By the 2080s, increasing parts of central Ontario, most of southern Quebec, and nearly 100% of the Maritimes, with the exception of Labrador, are predicted to be suitable habitat for Cx. pipiens. This amounts to a 1.5-fold increase in habitat suitability in the 2020s over currently predicted distributions, and a 2.5- and 5-fold increase in the 2050s and 2080s respectively. Under the more extreme scenario in the 2020s, projected range expansion is initially concentrated in the Maritime provinces and Newfoundland and Labrador as well as southern parts of Alberta. Expansion continues in these areas for the 2050s and 2080s but at a much slower rate than the moderate scenario predicted.
For Ae. albopictus, two out of the four studies that have modelled habitat suitability under future climate scenarios do so on a global scale.21,22 At this scale, it is difficult to obtain a detailed picture of distribution changes in Canada given the low resolution of the maps; but in general, both studies indicate potential continued expansion into western BC and southern Ontario, and new expansion into southern Alberta, southern Quebec, New Brunswick, Nova Scotia, Newfoundland, and southern Labrador.
A closer examination of Ae. albopictus range expansion can be found in two studies that model projections for the US and Canada specifically. Ogden et al. (2014) model habitat suitability for the 2050s and 2080s under moderate warming and extreme warming using three climactic indicators of Ae. albopictus survival: 1) overwintering conditions; 2) overwintering conditions combined with annual air temperature; and 3) an index of precipitation and air temperature suitability.19 Using either overwintering indicator, modest (~500 km) northward range expansion is projected along the BC coast by the 2050s under both the moderate and high emissions scenario. Using the precipitation and temperature index, greater northward range expansion (~600–1000 km) is projected along the BC coast and in the southern parts of eastern Canada by the 2050s under moderate warming. Range is also expected to expand into some northern parts of the Prairies and in the eastern foothills of the southern Rocky Mountains by the 2050s under extreme warming. Khan et al. (2020) also model Ae. albopictus habitat suitability under both a moderate and extreme warming scenario, extending predictions to the year 2100.20 They do not assess specific climactic indicators in their study, but similarly predict expansion of suitable habitat into southern parts of eastern Canada (under moderate warming) and coastal BC (under extreme warming).
Although each of these six studies demonstrates the impact of climate change on the distribution of these three mosquito vectors, a number of gaps remain in predicting future distribution. These include factors such as how climate change will affect host-vector interactions, predation by other species, competition with other species, and the potential adaptive capacity of the species itself, among other factors. Future models addressing how these factors will affect the ecology of WNV and distribution of vector species are needed.
How will Canada’s changing climate impact outbreaks of WNV in Canada?
Aside from geographic spread, climate-change-driven trends or events can impact WNV transmission through complex interactions between mosquitoes, viruses, and hosts. While these pathways have been widely studied, they vary by mosquito species (and life cycle) and can be influenced by a wide variety of environmental factors, such as land use, microclimates, topography, soils, infrastructure, and human population density. Consequently, results have often differed from study to study on the impact of temperature, precipitation, or relative humidity. This section summarizes the evidence on the impact of annual and seasonal warming, extreme heat, drought, and heavy precipitation on human cases of WNV.
Annual and seasonal warming
While the intensity of WNV transmission is typically kept in check at the confines of northern latitudes by cooler temperatures, there is ample evidence that above-average annual and seasonal temperatures in these regions is leading to sizeable changes in seasonal vector abundance, shifting transmission of these diseases further north.40,41 WNV transmission season in Canada is typically considered to be May through September. However, seasonal warming outside of this period is allowing mosquito populations and pathogen levels to develop earlier in the year, and extend the transmission season later into the fall. For example, warmer and shorter winters allow for greater survival of overwintering mosquitoes,42,43 while warmer spring weather allows mosquito populations and pathogen levels to develop more quickly and become more prevalent.44-46 Both warmer than average winters and springs have been associated with increased WNV incidence in humans in the contiguous US and Europe, as well as on more local scales, in New York, Connecticut, and Ontario.13,43,47-50 Warmer than average winter temperatures can also impact bird migration, breeding, and overall mortality,51,52 which could impact the WNV disease transmission cycle.
During the summer season, above-average seasonal temperatures have been linked to WNV epidemics in 2002–2004, and minimum summer temperatures have been found to predict human cases of the disease.46,47,53 While some studies have suggested that too-high summer temperatures have resulted in mosquito mortality, and thus represent a “natural check on viral amplification,” Chen et al. find that mean monthly summer temperatures in the Canadian Prairies are not likely to exceed the upper threshold of mosquito survival; as a result, increased summer temperatures will likely lead to greater vector development rates without a compensatory increase in mosquito mortality.17
Finally, for the fall, Hahn et al. (2015) found a small but significant increase in WNV incidence for the US nationally for each standard deviation increase in autumn temperature, suggesting an extension of the WNV transmission season. In the Upper Midwest and Northern Rockies/Plains region in particular, the odds of WNV incidence approximately doubled between October through to December.54 However, a study by Shocket et al. (2020) suggests that human behaviour may partially compensate for the expected effects of increased temperature on mosquitoes and hosts.40 For instance, mosquitoes may have less opportunity to bite humans as people tend to expose less skin and spend less time outdoors with the start of the school season and reduced daylight hours.40
Extreme heat
Definitions of extreme heat vary by region, but it is generally defined as summertime temperatures that are much hotter or more humid than average. There are several biological mechanisms that contribute to the association between extreme heat and increased WNV incidence. For both Culex and Aedes spp., high temperatures help increase mosquito abundance and accelerate mosquito development, but they also help to rapidly amplify viral replication in mosquitoes.46,55 Together, these changes directly affect the likelihood of the mosquito reaching maturity and increase their competence for infecting other hosts, thereby heightening the risk of disease spread.56-60 However, a too-high temperature (≥ 35°C) can have detrimental effects, greatly inhibiting mosquito development and decreasing mosquito survival, especially if such temperatures persist over several days.61 This inhibitive effect may, however, be mediated by the onset of the heat wave. As Jia et al. (2019) found, heat events emerging in late July did not influence the mosquito population as much as events earlier or later in the summer, suggesting the ability of mosquitoes to naturally adapt to more intense summer heat over the course of the season.61
Extreme heat events have also been linked to WNV outbreaks globally.58,62-64 The timing of WNV outbreaks following an extreme heat event appears to follow a geographic latitudinal gradient. That is, southern countries, which tend to have warmer climates, typically exhibit a more immediate increase in WNV cases following an extreme heat event, while northern countries, with colder climates, experience a lag effect, with cases increasing ~4 weeks following an extreme heat event.63 This lag effect is supported across numerous studies from North America.46,65-67 For example, in the US, Soverow et al. (2009) found mean weekly maximum temperatures to be positively and significantly associated with human WNV incidence during the same week and in the subsequent three weeks.68 Furthermore, mean weekly temperature increases of 5°C were associated with a 32–50% higher incidence of reported WNV cases.68 In Canada, Chen et al. (2013) showed that above-average mean monthly temperatures for June, July, and August were positively and significantly associated with human WNV incidence one month later.17
Finally, it is important to recognize differences between urban and rural areas with regard to extreme heat and WNV incidence. Dense, urbanized areas are particularly favourable WNV hotspots, made even more favourable given the additive impact of urban heat island effects on temperature.60 As a result, many outbreaks of WNV globally have and will continue to occur in urban areas without action to address urban heat islands.
Drought
Droughts have been linked with increased WNV incidence in numerous studies globally.3,66,69-77 In the US, a large national study determined drought to be the primary climatic driver of increased WNV epidemics, rather than within-season or winter temperatures.3 However, association between drought and WNV depends largely on the vector species affected, land cover composition, and the particular sequence of drought and precipitation that precedes WNV transmission season.78,79
For example, Cx. pipiens-restuans, the primary WNV vector in the eastern US and Canada (north of 36 degrees latitude), thrive in drought conditions, which helps to create breeding grounds high in organic matter (a result of drying content).71 Heavy rainfall events can negatively impact this species by flushing catch basins that are typically used by Cx. pipiens-restuans for egg-laying and diluting the organic content in other potential breeding sites.36,80,81 Moreover, it has been shown that Cx. pipiens-restuans can retain their eggs during a drought, allowing them time to locate the remaining water sources necessary for egg-laying, and which are often associated with human populations and WNV-competent bird species (e.g., bird baths, backyard ponds, etc.).82 In contrast, Cx. tarsalis, the primary WNV vector in the Western US and Canada, prefers water with lower organic content for breeding.71 Consequently, its population size is decreased during drought conditions, as a result of reduced availability of breeding habitats.64,83 Ae. albopictus differs from both Cx. pipiens-restuans and Cx. tarsalis in that its larval populations can flourish in very small pockets of water in either natural or human-made containers (e.g., discarded plastic containers). Unlike Cx. tarsalis, droughts do not have an outsized effect on Ae. albopictus because container-water sources are widespread, especially in urban areas. Moreover, eggs of container-breeding mosquitoes, like Ae. albopictus, are able to withstand drought conditions as an egg, and hatch when later submerged.84,85
While these divergent responses to drought across mosquito species may increase or decrease mosquito abundance, this does not always translate to a respective increase or decrease in human WNV incidence. Several other environmental factors can alter this association. Two such examples are mosquito predator and competitor species and associated land cover. In a study of natural wetlands in north-west Pennsylvania, researchers found that in wetlands that never dry (permanent wetland), predators will limit mosquito populations, whereas in wetlands that dry yearly (temporary wetland), competitors have adapted to this predictable drying and will similarly limit mosquito populations.38 However, in wetlands that dry during drought years only (semi-permanent wetland), mosquito predators and competitors are eliminated and the population of wetland mosquitoes can increase unchecked. Another study in the northeast and mid-western US supports this finding: in counties with a high proportional area of semi-permanent wetland, drought conditions resulted in an over 300% higher annual WNV incidence than in counties with a lower proportional area of semi-permanent wetland.76
Droughts can also cause changes to avian host abundance and habitat. For some species of wild birds, droughts have been shown to reduce populations, thereby increasing the likelihood of virus amplification in the remaining population.86 Water scarcity has also been shown to bring avian hosts and mosquitoes into closer contact through congregation near remaining water sources, increasing the cycling of the virus between mosquito and bird.87 Finally, the sequence of drought and precipitation can also impact human WNV incidence. In the above example, rainfall after a prolonged drought would likely result in the dispersal of avian hosts further from the water source, leading to greater risk of uninfected mosquitoes acquiring the virus.87 Rainfall events after drought further allow mosquitoes to lay their eggs and synchronize a cohort of host-seeking mosquitoes, allowing potentially WNV-infected mosquitoes to spread the virus to humans.88
Heavy rainfall
Heavy rainfall refers to instances during which the amount of rain in a location significantly exceeds what is normal for the area and time of year. In general, rainfall has two primary influences on the mosquito life cycle for Culex and Aedes spp. First, the increased humidity associated with rainfall enhances mosquito flight activity and host-seeking behavior. Secondly, increased rainfall can alter the quantity and type of aquatic habitat (nutrient-rich vs. nutrient-poor) available for breeding and larvae development.89,90 Depending on the mosquito species, this latter influence may increase mosquito abundance (e.g., Cx. tarsalis) or decrease mosquito abundance (Cx. pipiens),91-93 though a few studies have found precipitation to be unrelated to subsequent mosquito abundance in certain regions.92,94 Habitat type also likely plays a mediating role. In urban areas, which often have more impervious surfaces, heavy rainfall can wash out larvae from breeding sites; in contrast, heavy rainfall in rural areas, which have more permeable surfaces, can provide the moisture necessary for the development of breeding sites.92
Despite these general patterns, discerning the impact of heavy rainfall on WNV disease risk from existing studies is difficult. One reason for this difficulty is the differences among vector species and their preferred habitats and land use, as mentioned above.66,89,92 Other reasons include the differences in the spatial and temporal scales of published studies,66 and the variable effects of frequency, strength, and timing of rainfall events.90 For example, studies examining the impact of heavy precipitation on WNV cases in the US report diverging results. In a US study of meteorological conditions associated with reported human WNV cases, one or more days per week of heavy precipitation (defined as ≥ 50 mm in a single day) was associated with a 33% increased incidence of cases during the same week, with an elevated incidence in the subsequent two weeks.68 Precipitation of less than 40 mm in a single day progressively weakened the association, indicating that strength of rainfall is an important factor influencing WNV disease risk. Studies conducted at smaller spatial scales, however, indicate varying results. For example, in a study of the 2016 WNV season in South Dakota, Davis et al. (2017) found a negative association between total daily precipitation and WNV cases in the following week, but found positive effects for WNV cases after two months. A study by Chen et al. (2013) in the Canadian Prairie provinces found increasing precipitation associated with a lower WNV infection rate,17 despite increasing Cx. tarsalis abundance, while a separate study in the same region found increasing average precipitation decreased the Cx. tarsalis infection rate two to six weeks later.95
These differences in findings may partly be explained by the geographic range of different mosquito vectors. Hahn et al. (2015) illustrate the opposing effect of increased total annual precipitation (> 100 mm above average) between the eastern US and the western US. With the exception of the Northern Rockies and Plains, all western regions that experienced an increased total annual precipitation were associated with an increased WNV disease incidence.48 A similar study accounting for geographic differences found that human outbreaks of WNV are preceded by above-average rainfall in the eastern United States and below-average rainfall in the western United States in the year prior, the inverse of the relationship Hahn et al. (2015) observed for rainfall measured annually for the study year.70 The results of both studies reflect the geographical distribution of the primary WNV vectors in the US, where Cx. pipiens-restuans is predominant in the northern US, Cx. quinquefasciatus in the southern states, and Cx. tarsalis in the plains and western US. In areas where multiple mosquito vectors are present, precipitation anomalies in either direction may provide the ideal breeding sites for one of the vectors, which may explain the divergent results of studies conducted at smaller spatial scales.48 With regard to the different relationships between increased average rainfall in the year prior compared to the year of, the most plausible explanation seems to point to the long-term effects of precipitation on mosquito population dynamics vs. the short-term effects of precipitation on mosquito activity.70
More research is necessary at a variety of temporal and spatial scales to discern the impact of heavy rainfall on WNV across Canada, as well as the influence of increased precipitation on specific mosquito vectors and subsequently, human WNV.
What steps might municipal mosquito control programs consider in response to these changes?
Despite what is known about the geographical spread of vector mosquitoes and how climate-change-induced events may affect their abundance and infectivity, it remains challenging to predict the timing and scale of human outbreaks of WNV in both endemic and new regions. Strengthening public health preparedness for WNV outbreaks is therefore critical, as is reinforcing proactive WNV control efforts under both the current and future impacts of climate change. With regard to control efforts, Integrated Mosquito Management (IMM) programs are widely considered the gold standard.96 IMM is an evidence-based strategy composed of five core components, all of which play a critical role in effective management. They include: 1) surveillance, mapping, and evidence-based determination of action thresholds for mosquito control; 2) physical control of mosquito habitats through manipulation; 3) source reduction, biological control, and the application of targeted insecticides; 4) monitoring insecticide efficacy and resistance; and 5) regularly engaging the public to promote awareness and help with source reduction (e.g., removing standing water from yards). These core components are important for addressing WNV risk now and in a changing climate.
The Public Health Agency of Canada currently oversees the national WNV surveillance system—a multi-species surveillance system focussing on human, dead bird, mosquito, and animal data.97 However, provinces and municipalities are responsible for the physical and biological control of mosquito populations. Decisions regarding these activities should be based on local surveillance data where feasible. Mosquito testing in particular provides a specific indication of both spatial and temporal risk for human infection, and can help inform proactive and reactive control measures as needed. However, as this requires significant human and financial resources, it may not be appropriate in some areas. Where this is the case, equine WNV surveillance data (which is the most consistently reported nationally) and dead bird surveillance can be used to help address this gap. Surveillance through citizen science may also be considered, and is an especially valuable approach in vast geographic areas.98 Citizen science also has the added benefit of education and awareness for the communities involved. Most importantly, a consolidated approach to sharing surveillance information between municipalities, provinces, public health agencies, and academia will be necessary to ensure a more comprehensive understanding of current and future risk.
Aside from surveillance, communities may also be mobilized for environmental management to reduce mosquito breeding sites. For example, environmental public health practitioners and those working in mosquito control for municipalities could encourage residents to regularly clean up garbage in their yards and regularly change out the water in bird baths to avoid creating mosquito breeding sites. Increasing public awareness about mosquito-borne disease and how to reduce the risk of getting bitten is also central to both prevention of outbreaks and mitigation, should one occur.
In places where WNV is not yet a risk, but likely will be given the expansion of WNV vectors across Canada, continual surveillance focussed on the presence of specific mosquito species, their abundance, and whether WNV has entered the mosquito population will be important, as is monitoring for trends in neighbouring regions, irrespective of national or provincial borders. For Ae. albopictus in particular, both maritime sea transport and ground transportation have been identified as important dispersal pathways for the species into new countries.99 As such, vector surveillance and control at points of entry into Canada may be a useful strategy for limiting the species’ spread, as well as preventing other invasive species from being introduced.
Summary
Habitat suitability for Cx. pipiens, Cx. restuans, Cx. tarsalis and Ae. albopictus is predicted to expand as a result of Canada’s warmer climate and more frequent and extreme weather events. As a result, WNV is likely to emerge in areas where previously no risk existed, and increase in areas where risk was once low. Climate-change-induced events such as increased annual and seasonal temperature, heat waves, droughts, and heavy periods of rainfall may also amplify WNV risk, though timing and location are critical determinants among others that modulate risk. More research is needed to determine the current and future habitat of these four species in Canada, as well as the impacts of specific climate-change-induced trends on WNV risk in the Canadian context. As extreme weather events are expected to become more frequent, it is important to reinforce WNV control and monitoring efforts in places where the virus is already established, and enhance monitoring in places where it is possible to become established.100 Environmental public health professionals should work with health providers to communicate where WNV-positive vector populations are increasing to enable more timely diagnoses when a patient presents with symptoms to their physician. Surveillance at points of entry into Canada is also needed to limit the spread of non-native vectors into Canada. Finally, a consolidated approach to sharing information across Canada is also necessary to minimize risk and increase preparedness, should an outbreak occur. This will require increased communication between municipal, provincial, and academic partners involved in surveillance activities, and priority setting among these groups to establish future surveillance initiatives.
Acknowledgements
The author would like to thank NCCEH staff Michele Wiens, for literature search and referencing support, and Drs. Anne-Marie Nicol and Lydia Ma for their review of this document. The author would also like to thank the valued external reviewers who provided input on this document: Drs. Daniel Peach (University of Georgia), Atanu Sarkar (Memorial University), and John Soghigian (University of Calgary).
References
- Paz S. Climate change impacts on West Nile virus transmission in a global context. Phil Trans R Soc B. 2015;370(1665):20130561. Available from: https://royalsocietypublishing.org/doi/abs/10.1098/rstb.2013.0561
- Symptoms of West Nile virus. Ottawa, ON: Government of Canada; 2015 [Oct 31, 2022]; Available from: https://www.canada.ca/en/public-health/services/diseases/west-nile-virus/symptoms-west-nile-virus.html.
- Paull SH, Horton DE, Ashfaq M, Rastogi D, Kramer LD, Diffenbaugh NS, et al. Drought and immunity determine the intensity of West Nile virus epidemics and climate change impacts. Proc R Soc Lond B Biol Sci. 2017;284(1848):20162078. Available from: https://doi.org/10.1098/rspb.2016.2078.
- Ludwig A, Zheng H, Vrbova L, Drebot M, Iranpour M, Lindsay L. Climate change and infectious diseases: the challenges: increased risk of endemic mosquito-borne diseases in Canada due to climate change. Can Commun Dis Rep. 2019;45(4):91. Available from: https://doi.org/10.14745%2Fccdr.v45i04a03.
- Harrigan RJ, Thomassen HA, Buermann W, Smith TB. A continental risk assessment of West Nile virus under climate change. Glob Chang Biol. 2014;20(8):2417-25. Available from: https://doi.org/10.1111/gcb.12534.
- Ogden NH, Bouchard C, Brankston G, Brown EM, Corrin T, Dibernardo A, et al. Health of Canadians in a changing climate: advancing our knowledge for action. Ottawa, ON: Government of Canada; 2022. Available from: https://changingclimate.ca/health-in-a-changing-climate/chapter/6-0/.
- Pley C, Evans M, Lowe R, Montgomery H, Yacoub S. Digital and technological innovation in vector-borne disease surveillance to predict, detect, and control climate-driven outbreaks. The Lancet Planetary Health. 2021;5(10):e739-e45. Available from: https://doi.org/10.1016/S2542-5196(21)00141-8.
- National Collaborating Centre for Infectious Diseases. A short review of literature on the effects of climate change on mosquito-borne illnesses in Canada. Winnipeg, MB: NCCID; 2016. Available from: https://nccid.ca/publications/review-of-literature-on-effects-of-climate-change-on-mosquito-borne-illnesses-in-canada/.
- Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 2019;1436(1):157-73. Available from: https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/nyas.13950.
- Giordano B, Gasparotto A, Liang P, Nelder M, Russell C, Hunter F. Discovery of an Aedes (Stegomyia) albopictus population and first records of Aedes (Stegomyia) aegypti in Canada. Med Vet Entomol. 2020;34(1):10-6. Available from: https://doi.org/10.1111/mve.12408.
- Moua Y, Kotchi SO, Ludwig A, Brazeau S. Mapping the habitat suitability of West Nile Virus vectors in Southern Quebec and Eastern Ontario, Canada, with species distribution modeling and satellite earth observation data. Remote Sens. 2021;13(9):1637. Available from: https://www.mdpi.com/2072-4292/13/9/1637.
- Peach DA, Matthews BJ. The invasive mosquitoes of Canada: An entomological, medical, and veterinary review. Am J Trop Med. 2022;107(2):231. Available from: https://doi.org/10.4269/ajtmh.21-0167.
- Sergeev A, Lalonde C, Pons W. The role of climate change in the spread of vectors and vector-borne disease in Windsor-Essex County. Environ Health Rev. 2022;65(3):95-101.
- Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25(4):635-48. Available from: https://doi.org/10.1128/cmr.00045-12.
- Todoric D, Vrbova L, Mitri ME, Gasmi S, Stewart A, Connors S, et al. An overview of the National West Nile Virus Surveillance System in Canada: A One Health approach. CCDR. 2022;48(5):181.
- Ciota AT. West Nile virus and its vectors. Curr Opin Insect Sci. 2017;22:28-36. Available from: https://doi.org/10.1016/j.cois.2017.05.002.
- Chen CC, Jenkins E, Epp T, Waldner C, Curry PS, Soos C. Climate change and west nile virus in a highly endemic region of North America. Int J Environ Res Public Health. 2013;10(7):3052-71. Available from: https://doi.org/10.3390/ijerph10073052.
- Hongoh V, Berrang-Ford L, Scott M, Lindsay L. Expanding geographical distribution of the mosquito, Culex pipiens, in Canada under climate change. Appl Geogr. 2012;33:53-62. Available from: https://www.semanticscholar.org/paper/Expanding-geographical-distribution-of-the-Culex-in-Hongoh-Berrang%E2%80%90Ford/ec4436dc59d97421b28a59772fd7ea6ee3ba7789.
- Ogden NH, Milka R, Caminade C, Gachon P. Recent and projected future climatic suitability of North America for the Asian tiger mosquito Aedes albopictus. Parasit Vectors. 2014 Dec;7(1):532. Available from: https://doi.org/10.1186/s13071-014-0532-4.
- Khan SU, Ogden NH, Fazil AA, Gachon PH, Dueymes GU, Greer AL, et al. Current and projected distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S. Environ Health Perspect. 2020 May;128(5):57007. Available from: https://doi.org/10.1289/ehp5899.
- Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665):20140135. Available from: https://royalsocietypublishing.org/doi/abs/10.1098/rstb.2014.0135
- Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE. 2018;13(12):e0210122. Available from: https://doi.org/10.1371/journal.pone.0210122.
- Hongoh V, Berrang-Ford L, Ogden N, Lindsay R, Scott M, Artsob H. A review of environmental determinants and risk factors for avian-associated mosquito arboviruses in Canada. Biodivers. 2009;10(2-3):83-91. Available from: https://doi.org/10.1080/14888386.2009.9712849.
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Gainesville, FL: University Press Florida; 2005. Available from: https://upf.com/book.asp?id=9780813062334.
- Darsie Jr RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Washington, DC: Walter Reed Army Inst of Research 1981. Available from: https://upf.com/book.asp?id=9780813062334.
- Mattingly PF. The Culex pipiens complex. Trans Royal Entomol Soc London. 1951;102(pt. 7). Available from: https://www.cabdirect.org/cabdirect/abstract/19521000130.
- Gorris ME, Bartlow AW, Temple SD, Romero-Alvarez D, Shutt DP, Fair JM, et al. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit Vectors. 2021 Oct 23;14(1):547. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-021-05051-3.
- Stuart T. An Overview of the West Nile Virus and California Serogroup of Vector Competent Mosquito Species in the Northwest Territories from 2004-2018. Available from: https://www.enr.gov.nt.ca/sites/enr/files/resources/an_overview_of_the_west_nile_virus_and_california_serogroup_of_vector_competent_mosquito_species_in_the_northwest_territories_from_2004-2018.pdf.
- Peach DA. First record of Culex tarsalis (Diptera: Culicidae) in the Yukon. J Entomol Soc BC. 2018;115:123-5. Available from: https://journal.entsocbc.ca/index.php/journal/article/view/1006.
- Peach DA, Poirier LM. New distribution records and range extensions of mosquitoes in British Columbia and the Yukon Territory. bioRxiv. 2020. Available from: https://doi.org/10.1101/2020.01.24.919233.
- Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4:e08347. Available from: https://doi.org/10.7554/elife.08347.
- Ding F, Fu J, Jiang D, Hao M, Lin G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 2018;178:155-62. Available from: https://doi.org/10.1016/j.actatropica.2017.11.020.
- Zhang X, Flato G, Kirchmeier-Young M, Vincent L, Wan H, Wang X, et al. Changes in temperature and precipitation across Canada; Chapter 4. In: Bush E, Lemmen DS, editors. Canada’s changing climate report. Ottawa, ON: Government of Canada; 2019. p. 112-93. Available from: https://changingclimate.ca/CCCR2019/chapter/4-0/.
- Intergovernmental Panel on Climate Change (IPCC) 2022: Summary for policymakers [H.-O. Pörtner, D.C. Roberts, E.S. Poloczanska, K. Mintenbeck, M. Tignor, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem (eds.)]. Climate Change 2022: Impacts, adaptation and vulnerability Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H-O Pörtner, DC Roberts, M Tignor, ES Poloczanska, K Mintenbeck, A Alegría, M Craig, S Langsdorf, S Löschke, V Möller, A Okem, B Rama (eds)]. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2022. p. 3-33. Available from: https://doi.org/10.1017/9781009325844.001.
- Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6(1):1-12. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/1756-3305-6-351.
- Koenraadt C, Harrington L. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J Med Entomol. 2008;45(1):28-35. Available from: https://doi.org/10.1093/jmedent/45.1.28.
- Shaman J, Stieglitz M, Stark C, Le Blancq S, Cane M. Using a dynamic hydrology model to predict mosquito abundances in flood and swamp water. Emerg Infect Dis. 2002;8(1):8. Available from: https://doi.org/10.3201%2Feid0801.010049.
- Chase JM, Knight TM. Drought‐induced mosquito outbreaks in wetlands. Ecol Lett. 2003;6(11):1017-24. Available from: https://doi.org/10.1046/j.1461-0248.2003.00533.x.
- Johnson B, Sukhdeo M. Drought-induced amplification of local and regional West Nile virus infection rates in New Jersey. J Med Entomol. 2013;50(1):195-204.
- Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F, et al. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23 C and 26 C. eLife. 2020;9:e58511. Available from: https://doi.org/10.7554%2FeLife.58511.
- Vogels CB, Hartemink N, Koenraadt CJ. Modelling West Nile virus transmission risk in Europe: effect of temperature and mosquito biotypes on the basic reproduction number. Sci Rep. 2017;7(1):1-11. Available from: https://doi.org/10.1038/s41598-017-05185-4.
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7(4):742. Available from: https://doi.org/10.3201/eid0704.010426.
- Marini G, Manica M, Delucchi L, Pugliese A, Rosà R. Spring temperature shapes West Nile virus transmission in Europe. Acta Trop. 2021;215:105796. Available from: https://doi.org/10.1016/j.actatropica.2020.105796.
- Gardner AM, Hamer GL, Hines AM, Newman CM, Walker ED, Ruiz MO. Weather variability affects abundance of larval Culex (Diptera: Culicidae) in storm water catch basins in suburban Chicago. J Med Entomol. 2012;49(2):270-6. Available from: https://doi.org/10.1603%2Fme11073.
- Chuang T-W, Knepper RG, Stanuszek WW, Walker ED, Wilson ML. Temporal and spatial patterns of West Nile virus transmission in Saginaw County, Michigan, 2003–2006. J Med Entomol. 2011;48(5):1047-56. Available from: https://doi.org/10.1603/me10138.
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol. 2014;43(2):309-17. Available from: https://doi.org/10.1093/jmedent/43.2.309.
- Keyel AC, Elison Timm O, Backenson PB, Prussing C, Quinones S, McDonough KA, et al. Seasonal temperatures and hydrological conditions improve the prediction of West Nile virus infection rates in Culex mosquitoes and human case counts in New York and Connecticut. PLoS ONE. 2019;14(6):e0217854. Available from: https://doi.org/10.1371/journal.pone.0217854.
- Hahn MB, Monaghan AJ, Hayden MH, Eisen RJ, Delorey MJ, Lindsey NP, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med. 2015;92(5):1013. Available from: https://doi.org/10.4269%2Fajtmh.14-0737.
- Wijayasri S, Nelder M, Russell C, Johnson K, Johnson S, Badiani T, et al. Challenges in infection control: West Nile virus illness in Ontario, Canada: 2017. Can Commun Dis Rep. 2019;44(1):32. Available from: https://doi.org/10.14745%2Fccdr.v45i01a04.
- Wimberly MC, Lamsal A, Giacomo P, Chuang T-W. Regional variation of climatic influences on West Nile virus outbreaks in the United States. Am J Trop Med. 2014;91(4):677. Available from: https://doi.org/10.4269%2Fajtmh.14-0239.
- Both C, Bouwhuis S, Lessells C, Visser ME. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441(7089):81-3. Available from: https://www.nature.com/articles/nature04539.
- Culp LA, Cohen EB, Scarpignato AL, Thogmartin WE, Marra PP. Full annual cycle climate change vulnerability assessment for migratory birds. Ecosphere. 2017;8(3):e01565. Available from: https://doi.org/10.1002/ecs2.1565.
- Watts MJ, i Monteys VS, Mortyn PG, Kotsila P. The rise of West Nile Virus in Southern and Southeastern Europe: a spatial–temporal analysis investigating the combined effects of climate, land use and economic changes. One Health. 2021;13:100315. Available from: https://doi.org/10.1016/j.onehlt.2021.100315.
- Hahn MB, Monaghan AJ, Hayden MH, Eisen RJ, Delorey MJ, Lindsey NP, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med. 2015;92(5):1013. Available from: https://doi.org/10.4269%2Fajtmh.14-0737.
- Rios M. Climate change and vector‐borne viral diseases potentially transmitted by transfusion. ISBT Sci Ser. 2009;4(1):87-94. Available from: https://doi.org/10.1098%2Frstb.2013.0552.
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4(6):e1000092. Available from: https://doi.org/10.1371/journal.ppat.1000092.
- Liu Z, Zhang Z, Lai Z, Zhou T, Jia Z, Gu J, et al. Temperature increase enhances Aedes albopictus competence to transmit dengue virus. Front Microbiol. 2017;8:2337. Available from: https://doi.org/10.3389/fmicb.2017.02337.
- Epstein PR, Defilippo C. West Nile virus and drought. Glob Chang Hum Health. 2001;2(2):105-7. Available from: http://dx.doi.org/10.1023/A:1015089901425.
- Dohm DJ, O'Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39(1):221-5. Available from: https://doi.org/10.1093/jmedent/43.2.309.
- Paz S, Albersheim I. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile Fever outbreaks (Israeli case study: 2001–2005). Ecohealth. 2008;5(1):40-8. Available from: https://doi.org/10.1007/s10393-007-0150-0.
- Jia P, Liang L, Tan X, Chen J, Chen X. Potential effects of heat waves on the population dynamics of the dengue mosquito Aedes albopictus. PLoS Negl Trop Dis. 2019;13(7):e0007528. Available from: https://doi.org/10.1371/journal.pntd.0007528.
- Paz S. The West Nile Virus outbreak in Israel (2000) from a new perspective: the regional impact of climate change. Int J Environ Res Public Health. 2006;16(1):1-13. Available from: https://doi.org/10.1080/09603120500392400.
- Paz S, Malkinson D, Green MS, Tsioni G, Papa A, Danis K, et al. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS ONE. 2013;8(2):e56398. Available from: https://doi.org/10.1371/journal.pone.0056398.
- Reisen WK, Cayan D, Tyree M, Barker CM, Eldridge B, Dettinger M. Impact of climate variation on mosquito abundance in California. J Vector Ecol. 2008;33(1):89-98. Available from: https://doi.org/10.3376/1081-1710(2008)332.0.co;2.
- Chen C-C, Epp T, Jenkins E, Waldner C, Curry PS, Soos C. Modeling monthly variation of Culex tarsalis (Diptera: Culicidae) abundance and West Nile Virus infection rate in the Canadian Prairies. Int J Environ Res Public Health. 2013;10(7):3033-51. Available from: https://doi.org/10.3390%2Fijerph10073033.
- Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 2010;3(1):1-16. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/1756-3305-3-19.
- Kunkel KE, Novak RJ, Lampman RL, Gu W. Modeling the impact of variable climatic factors on the crossover of Culex restauns and Culex pipiens (Diptera: Culicidae), vectors of West Nile virus in Illinois. Am J Trop Med. 2006;74(1):168-73. Available from: https://pubmed.ncbi.nlm.nih.gov/16407364/.
- Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. Infectious disease in a warming world: how weather influenced West Nile Virus in the United States (2001-2005). Environ Health Perspect. 2009;117(7):1049-52. Available from: https://doi.org/10.1289%2Fehp.0800487.
- Wang G, Minnis RB, Belant JL, Wax CL. Dry weather induces outbreaks of human West Nile virus infections. BMC Infect Dis. 2010;10(1):1-7. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-10-38.
- Landesman WJ, Allan BF, Langerhans RB, Knight TM, Chase JM. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7(3):337-43. Available from: https://doi.org/10.1089/vbz.2006.0590.
- Shaman J, Day JF, Komar N. Hydrologic conditions describe West Nile virus risk in Colorado. Int J Environ Res Public Health. 2010;7(2):494-508. Available from: https://doi.org/10.3390/ijerph7020494.
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42(2):134-41. Available from: https://doi.org/10.1093/jmedent/42.2.134.
- Shaman J, Harding K, Campbell SR. Meteorological and hydrological influences on the spatial and temporal prevalence of West Nile virus in Culex mosquitoes, Suffolk County, New York. J Med Entomol. 2011;48(4):867-75. Available from: https://doi.org/10.1603/me10269.
- Camp JV, Nowotny N. The knowns and unknowns of West Nile virus in Europe: what did we learn from the 2018 outbreak? Expert Rev Anti Infect Ther. 2020;18(2):145-54. Available from: https://doi.org/10.1080/14787210.2020.1713751.
- Marcantonio M, Rizzoli A, Metz M, Rosà R, Marini G, Chadwick E, et al. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS ONE. 2015;10(3):e0121158. Available from: https://doi.org/10.1371/journal.pone.0121158.
- Skaff NK, Cheruvelil KS. Fine-scale wetland features mediate vector and climate-dependent macroscale patterns in human West Nile virus incidence. Landsc. 2016;31(7):1615-28. Available from: https://link.springer.com/article/10.1007/s10980-016-0346-1.
- Johnson BJ, Munafo K, Shappell L, Tsipoura N, Robson M, Ehrenfeld J, et al. The roles of mosquito and bird communities on the prevalence of West Nile virus in urban wetland and residential habitats. Urban Ecosyst. 2012;15(3):513-31. Available from: https://doi.org/10.1007%2Fs11252-012-0248-1.
- Kim EJ. The impacts of climate change on human health in the United States: A scientific assessment, by us global change research program. J Am Plann Assoc. 2016;82(4):418-9. Available from: https://health2016.globalchange.gov/low/ClimateHealth2016_FullReport_small.pdf.
- Ezenwa VO, Milheim LE, Coffey MF, Godsey MS, King RJ, Guptill SC. Land cover variation and West Nile virus prevalence: patterns, processes, and implications for disease control. Vector Borne Zoonotic Dis. 2007;7(2):173-80. Available from: https://doi.org/10.1089/vbz.2006.0584.
- Geery P, Holub R. Seasonal abundance and control of Culex spp. in catch basins in Illinois. J Am Mosq Control Assoc. 1989;5(4):537-40. Available from: https://pubmed.ncbi.nlm.nih.gov/2614404/.
- Reisen W. Ecology of West Nile virus in North America. Viruses. 2013;5:2079–105. Available from: https://doi.org/10.3390%2Fv5092079.
- Johnson BJ, Fonseca DM. The effects of forced-egg retention on the blood-feeding behavior and reproductive potential of Culex pipiens (Diptera: Culicidae). J Insect Physiol. 2014;66:53-8. Available from: https://doi.org/10.1016/j.jinsphys.2014.05.014.
- Wimberly MC, Hildreth MB, Boyte SP, Lindquist E, Kightlinger L. Ecological niche of the 2003 West Nile virus epidemic in the northern Great Plains of the United States. PLoS ONE. 2008;3(12):e3744. Available from: https://doi.org/10.1371/journal.pone.0003744.
- Vitek CJ, Livdahl T. Hatch plasticity in response to varied inundation frequency in Aedes albopictus. J Med Entomol. 2009;46(4):766-71. Available from: https://doi.org/10.1603/033.046.0406.
- Benelli G, Wilke AB, Beier JC. Aedes albopictus (Asian tiger mosquito). Trends Parasitol. 2020. Available from: https://doi.org/10.1016/j.pt.2020.01.001.
- Albright TP, Pidgeon AM, Rittenhouse CD, Clayton MK, Flather CH, Culbert PD, et al. Effects of drought on avian community structure. Global Change Biology. 2010;16(8):2158-70. Available from: https://doi.org/10.1111/j.1365-2486.2009.02120.x.
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg Infect Dis. 2002;8(6):575. Available from: https://doi.org/10.3201%2Feid0806.010417.
- Day JF, Curtis GA, Edman JD. Rainfall-directed oviposition behavior of Culex nigripalpus (Diptera: Culicidae) and its influence on St. Louis encephalitis virus transmission in Indian River County, Florida. J Med Entomol. 1990;27(1):43-50. Available from: https://doi.org/10.1093/jmedent/27.1.43.
- Shaman J, Day JF. Reproductive phase locking of mosquito populations in response to rainfall frequency. PLoS ONE. 2007;2(3):e331. Available from: https://doi.org/10.1371%2Fjournal.pone.0000331.
- Shand L, Brown WM, Chaves LF, Goldberg TL, Hamer GL, Haramis L, et al. Predicting West Nile virus infection risk from the synergistic effects of rainfall and temperature. J Med Entomol. 2016;53(4):935-44. Available from: https://doi.org/10.1093/jme/tjw042.
- Chuang T-W, Hildreth MB, Vanroekel DL, Wimberly MC. Weather and land cover influences on mosquito populations in Sioux Falls, South Dakota. J Med Entomol. 2011;48(3):669-79. Available from: https://doi.org/10.1603/me10246.
- Beard CB, Eisen RJ, Barker C, Garofalo J, Hahn M, Hayden M, et al. Ch. 5: vectorborne diseases: US Global Change Research Program, Washington, DC; 2016. Available from: https://health2016.globalchange.gov/vectorborne-diseases.
- Jones CE, Lounibos LP, Marra PP, Kilpatrick AM. Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the mid-Atlantic United States. J Med Entomol. 2012;49(3):467-73. Available from: https://doi.org/10.1603/me11191.
- Crowder DW, Dykstra EA, Brauner JM, Duffy A, Reed C, Martin E, et al. West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PLoS ONE. 2013;8(1):e55006. Available from: https://doi.org/10.1371/journal.pone.0055006.
- Chen C-C, Epp T, Jenkins E, Waldner C, Curry PS, Soos C. Predicting weekly variation of Culex tarsalis (Diptera: Culicidae) West Nile virus infection in a newly endemic region, the Canadian prairies. J Med Entomol. 2014;49(5):1144-53. Available from: https://doi.org/10.1603/me11221.
- Association AMC. Best practices for integrated mosquito management: a focused update. Sacramento, California, USA. 2017.
- Health Canada. Mosquito-borne diseases national surveillance report. Ottawa, ON: Government of Canada [updated 25 Feb 2022; accessed Oct 31, 2022]; Available from: https://www.canada.ca/en/public-health/services/diseases/west-nile-virus/west-nile-virus-other-mosquito-borne-disease.html.
- Sarkar A. Study of distribution of invasive mosquitoes in NL & St. Pierre/Miquelon: a citizen science approach [Webinar]. Ottawa, ON: Canadian Public Health Association 2021. Available from: https://www.youtube.com/watch?v=O-ouRy7biCQ.
- Swan T, Russell TL, Staunton KM, Field MA, Ritchie SA, Burkot TR. A literature review of dispersal pathways of Aedes albopictus across different spatial scales: implications for vector surveillance. Parasit Vectors. 2022;15(1):1-13. Available from: https://doi.org/10.1186/s13071-022-05413-5.
- Paz S. Effects of climate change on vector-borne diseases: an updated focus on West Nile virus in humans. Emerg Topics Life Sci. 2019;3(2):143-52. Available from: https://doi.org/10.1042/etls20180124.
- Nakicenovic N, Alcamo J, Davis G, Vries Bd, Fenhann J, Gaffin S, et al. Special report on emissions scenarios. 2000. Available from: https://www.ipcc.ch/site/assets/uploads/2018/03/sres-en.pdf.
- Intergovernmental Panel on Climate Change (IPCC), 2014: Climate change 2014: Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland 2014. Available from: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full.pdf.
Appendix A
Emissions scenarios: These are possible pathways developed by the IPCC in 2000 that society may take in the emission of greenhouse gases in the future.101 The scenarios fall under four storylines (A1, A2, B1, and B2) and consist of the following six scenarios:
A1 storyline: This storyline and scenario group describes “a future world of very rapid economic growth, global population that peaks in mid-century and declines thereafter, and the rapid introduction of new and more efficient technologies. Major underlying themes are convergence among regions, capacity building, and increased cultural and social interactions, with a substantial reduction in regional differences in per capita income. The A1 scenario family develops into three groups that describe alternative directions of technological change in the energy system.”101
- The A1F1 scenario depicts a fossil fuel intensive future.
- The A1B scenario depicts a balanced future split between both fossil fuels and non-fossil fuel technologies.
- The A1T scenario depicts a predominantly non-fossil fuel future.
A2 storyline: This storyline and scenario describes “a heterogeneous world. The underlying theme is self-reliance and preservation of local identities. Fertility patterns across regions converge very slowly, which results in continuously increasing global population. Economic development is primarily regionally oriented and per capita economic growth and technological change are more fragmented and slower than in other storylines.” 101 The A2 storyline has only one scenario, titled the A2 scenario.
B1 storyline: This storyline and scenario describes “a convergent world with the same global population that peaks in mid-century and declines thereafter, as in the A1 storyline, but with rapid changes in economic structures toward a service and information economy, with reductions in material intensity, and the introduction of clean and resource-efficient technologies. The emphasis is on global solutions to economic, social, and environmental sustainability, including improved equity, but without additional climate initiatives.”101 The B1 storyline has only one scenario, titled the B1 scenario.
B2 storyline: This storyline and scenario describes “a world in which the emphasis is on local solutions to economic, social, and environmental sustainability. It is a world with continuously increasing global population at a rate lower than A2, intermediate levels of economic development, and less rapid and more diverse technological change than in the B1 and A1 storylines. While the scenario is also oriented toward environmental protection and social equity, it focuses on local and regional levels.”101 The B2 storyline has only one scenario, titled the B2 scenario.
All storylines and scenarios were developed to be equally valid with no assigned probabilities of occurrence.
Representative concentration pathways (RCPs): These are greenhouse gas concentration trajectories adopted by the IPCC in 2014 for climate modelling and research.102 There are four pathways, each of which describe different climate futures: RCP2.6, RCP4.5, RCP6, and RCP8.5. The numbers refer to radiative forcings (global energy imbalances) that are measured in watts per square metre (W/m2), by the year 2100.
RCP2.6: This pathway is described by the IPCC as a low emission scenario characterized by active mitigation, with emissions peaking by 2020 before declining. It represents a peak in radiative forcing at ~ 3 W/m2 mid-century before declining to 2.6 W/m2 by 2100. RCP2.6 is projected to result in global temperature rise between 0.3°C and 1.7°C by 2100.
RCP4.5: This pathway is described by the IPCC as an intermediate emission scenario, with emissions peaking by 2040 before declining. It represents a stabilization in radiative forcing at 4.5 W/m2 post-2100. RCP4.5 is projected to result in global temperature rise between 1.1°C and 2.6°C by 2100.
RCP6: This pathway is described by the IPCC as an intermediate emission scenario, with emissions peaking by 2080 before declining. It represents a stabilization in radiative forcing at 6 W/m2 post-2100. RCP6 is projected to result in global temperature rise between 1.4°C and 3.1°C by 2100.
RCP8.5: This pathway is described by the IPCC as a high emission scenario, with emissions continuing to rise throughout the 21st century. It represents a rise in radiative forcing to 8.5 W/m2 in 2100. RCP8.5 is projected to result in global temperature rise between 2.6°C and 4.8°C by 2100.
Table A1. Projected climate scenarios used in each study
|
Study |
General or Regional Circulation Models |
Emissions Scenarios |
Representative Concentration Pathways |
|
17 |
NCAR-PCM |
A2 |
-- |
|
18 |
CGCM3 |
A2 |
-- |
|
19 |
CRCM4.2.3 |
A2 |
RCP 4.5 |
|
20 |
CanRCM4-CanESM2 CRCM5-CanESM2 CRCM5-MPI-ESM-LR HIRHAM5-EC-EARTH |
-- |
RCP4.5 |
|
21 |
BCCR-BCM 2.0 |
A2 |
-- |
Author
Leah Rosenkrantz is an EH and KT Scientist at NCCEH.








